3.1 THE PHOTOELECTRIC EFFECT:
What is the photoelectric effect?
- If radiation of high enough frequency is shone onto the surface of a metal, it will emit electrons instantly.
- For most metals, the required frequency is in the ultraviolet region.
- Because of the way atoms are bonded together in metals, metals contain free electrons that are able to move about in the metal.
- The electron near the surface of the metal absorbs energy from the radiation which makes them vibrate.
- If an electron absorbs enough energy, the bonds holding it can be broken and the electron can be released.
- This is the photoelectric effect and the electrons being emitted are called photoelectrons.
Main conclusions made from the photoelectric effect:
- For a given metal, no electrons are emitted if the radiation has a frequency below a certain value called the threshold frequency.
- Photoelectrons are emitted with a variety of kinetic energies from zero to a maximum value. (Value of maximum kinetic energy increases with the frequency of the radiation).
- Intensity of the radiation is the energy per second hitting an area of the metal. Maximum kinetic energy of the photoelectrons is unaffected by intensity of the radiation.
- Number of photoelectrons emitted per second is proportional to the intensity of the radiation, provided the the frequency is greater than the threshold frequency.
Problems:
- Minimum frequency depends upon the type of metal.
- Wavelength of the incident light must be less than a maximum value = to the speed of light divided by the threshold frequency.
- Photoelectric emission occurs without any delay, instant.
- As soon as the incident radiation is directed at the surface of the metal, provided the frequency exceeds the threshold frequency.
Threshold frequency:
- Wave theory says that for a particular frequency of EM wave, the energy carried should be proportional to the intensity of the beam.
- The energy carried by the EM wave would also spread evenly over the wavefront.
- This means that if an EM wave were shone on a metal, each free electron on the surface would gain a bit of energy from each incoming wavefront, gradually each electron would gain enough energy to escape the metal.
- The lower the frequency, the longer it takes for the electron to gain enough energy to escape, provided that the wave is above the threshold frequency.
Kinetic energy of the photoelectrons:
- Greater the intensity of the wave, the more energy it transfers to to each electron.
- The kinetic energy increases with intensity.
- Wave theory can’t explain the fact that the kinetic energy depends only on the frequency in the photoelectric effect.
Stopping potential:
- Electrons that escape from the metal plate can be pulled back by giving the metal plate a sufficient positive charge.
- Minimum potential needed to stop it is called the stopping potential (Vs)
- At this potential, the maximum kinetic energy of the emitted of the electron must do extra work equal to e × Vs to leave the metal surface.
- Hence maximum kinetic energy is = e × Vs
3.2 MORE ABOUT PHOTOELECTRICITY:
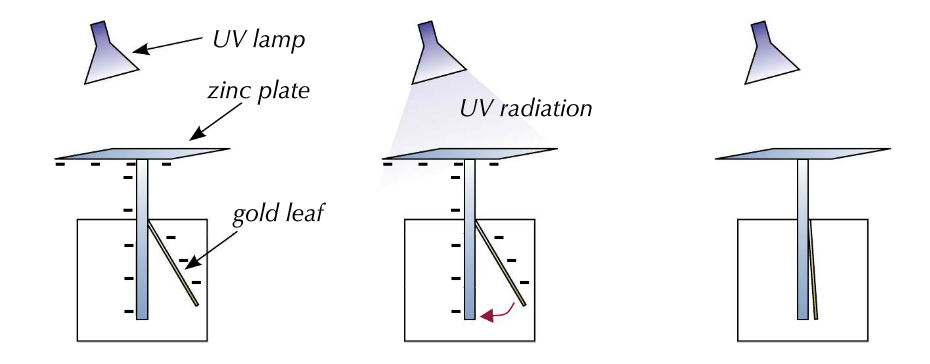
Practical demonstration of the photoelectric effect:
- Photoelectric effect is easily demonstrated:
- A zinc plate is attached to the top of an electroscope, a box with a piece of metal with a strip of gold leaf attached.
- The zinc plate is negatively charged, therefore the metal in the box is negatively charged.
- The negative charge repels the gold lead causing it to rise up.
- The UV light is shone onto the zinc plate.
- The energy of the light causes electrons to be lost from the zinc plate with the photoelectric effect.
- As the zinc plate and metal loses their negative charge, the gold lead if no longer repelled and therefore falls down.
Work function:
- When EM waves hits a metal, the metal’s surface is bombarded by photons.
- If one photon collides with a free electron, the electron will gain energy which is equal to hf (E=hf)
- Before an electron can leave the surface, it needs enough energy to break free.
- This energy is called the work function (Φ) – Dependant upon the metal.
- If the energy gained from the photon is greater than the work function energy, the electron can be emitted.
- If the energy is lower, the electron will shake a bit and release energy as a photon.
- The metal will heat but no electrons are emitted.
- Since for electrons to be released:
- Therefore, the threshold frequency must be:
Conduction electrons:
- Conduction electrons in a metal move randomly, like molecules of gas.
- Average kinetic energy of a conduction electron depends upon the temperature of the metal.
- Work function of a metal is the minimum energy need by a conduction electron to escape from the metal surface when the metal is at zero potential.
- The work function is of the order (10–19J) which is 20 times greater than the average kinetic energy of a conduction electron in a metal at 300 K
- When a conduction electron absorbs a photon, its kinetic energy increases by an amount equal to the energy of the photon.
- If the energy exceeds the work function of the metal, the conduction electron can leave the metal.
- If the electron does not leave the metal, it collides with the other electrons and positive ions and it quickly loses its kinetic energy.
The vacuum photocell:
- a vacuum photocell is a glass tube that contains a metal plate, referred to as the photocathode, and a smaller metal electrode referred to as the anode.
- The diagram below shows a vacuum photocell in a circuit, when light of a frequency greater than the threshold frequency for the metal is directed at the photocathode, electrons are emitted from the cathode and are attracted to the anode.
- The microammeter in the circuit can be used to measure the photoelectric current.
- Proportional to the number of electrons per second that transfer from the cathode to the anode.
- For a photoelectric current, I, the number of photoelectrons per second is I/e, where e is the charge of the electron.
- The photoelectric current is proportional to the intensity of the light incident on the cathode.
- The light intensity is measure of the energy per second carried by the light.
- Proportional to the number of photons per second incident on the cathode.
- Each electron must have absorbed one photon to escape the metal surface.
- Number of photoelectrons emitted per second is therefore proportional to the intensity of the light.
- The intensity of the incident light does not affect the kinetic energy of a photoelectron.
- No matter how intense the light is, the energy gained by a photoelectron is due to the absorption of one photon only.
- Therefore the formula for the maximum kinetic energy of a photoelectron is given by:
- The maximum kinetic energy of the photoelectrons emitted for a given frequency of light is measured using a photocell.
- The measurements for different frequencies are plotted as a graph of Ekmax against f, a straight line of the form, y=mx+c is obtained.
- This is in accordance with the photoelectric equation.

- The gradient gives the planck constant.
- The X intercept gives threshold frequency:
- The Y intercept gives the work function:
- (
)
- (

3.3 COLLISIONS OF ELECTRONS WITH ATOMS:
The Electron Volt:
- Defined as the kinetic energy carried by an electron after it has been accelerated from rest through a potential difference of 1 Volt.
- The energy gained by an electron (eV) is equal to the voltage (V)
- 1 eV =1.6 x 10–19 Joules
Ionisation:
- An ion is a charged atom.
- The number of electrons in an ion is equal to the number of protons.
- Formed from an uncharged atom by adding or removing electrons from the atom.
- Adding electrons = negative.
- Removing electrons = positive.
Any process of creating ions is called ionisation:
- Alpha, beta and gamma radiation create ions when they pass through substances and collide with the atoms of the substance.
- Electrons passing through a fluorescent tube create ions when they collide with the atoms of the gas or vapour.
Ionisation energy of a gas atom =eV
Excitation by collision:
- Using gas-filled tubes with a metal grid between the filaments and the anode, we can show that gas atoms can absorb energy from colliding electrons without actually being ionised.
- Process is known as excitation, which happens at certain energies.
- It is a characteristic of the atoms of the gas.
- If a colliding electron loses all its kinetic energy when it causes excitation, the current due to the flow of electrons through the gas is reduced.
- If the colliding electron does not have enough kinetic energy to cause excitation, it is deflected by the gas atoms with no overall loss of kinetic energy.
- The energy values at which an atom absorbs energy are known asexcitation energies which can be determined for the atoms in the gas-filled tube by increasing the potential difference between the filament and the anode and measuring the pd when the anode current falls.
- For example, two prominent excitation energies of a mercury atom are 4.9eV and 5.7 eV meaning that the current would fall at 4.9V and 5.7V
- When excitation occurs, the colliding electron makes an electron inside the atom move from an inner shell to an outer shell.
- Energy is needed for this process, because they atomic electron moves away from the nucleus of the atom.
- Excitation energy is always < ionisation energy of the atom, because the atomic electron is not removed completely when excitation occurs.
3.6 WAVE-PARTICLE DUALITY:
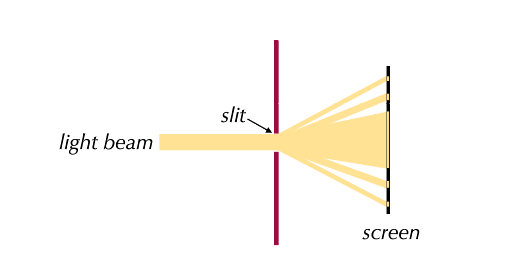
Diffraction:
- When a beam of light passes through a narrow gap, it spreads out, a scientific principle called diffraction.
- Diffraction can only be explained using waves.
- If light was a particle, then the light particles would either be too big or just pass straight through the gap and the beam would be unchanged.
Photoelectric effect:
- The particle-like-nature is observed in the photoelectric effect
- It proves that when light is directed at a metal surface, an electron at the surface is absorbed a photon of frequency f, the kinetic energy of the electron is increased by hf.
- The electron can escape if the energy it gains from the photon exceeds the work function of the metal.
Matter waves:
- If light has a dual wave-particle nature, particles of matter also have a dual wave-particle nature.
- Electrons in a beam can be deflected by a magnetic field.
- This is evidence that electrons have a particle-like nature.
- The idea that matter particles also have a wave-like nature was first considered by Louis de Broglie in 1923. (de Broglie hypothesis)
- By extending the ideas of duality from photons to matter particles, Broglie put forward an idea that:
- Matter particles have a dual wave-particle nature
- The wave-like behaviour of a matter particle is determined by the de Broglie Wavelength, λ, which is related to the momentum, p, of the particle by means of the following equation:
- The de Broglie wavelength of a particle can be changed by altering the velocity of the particle.
Evidence for de Broglie’s hypothesis:
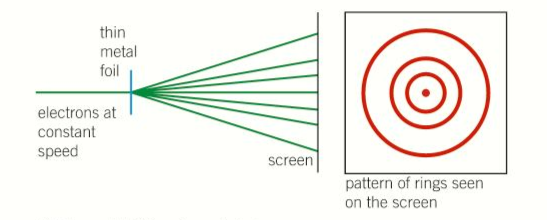
Electron diffraction:
- The wave-like nature of electrons was discovered when de Broglie put forward his hypothesis, which demonstrated that a beam of electrons can be diffracted
- The following diagram below shows hows this can be done.
How this can be achieved:
- A narrow beam of electrons in a vacuum tube is directed at a thin metal foil.
- A metal is made up of many tiny crystalline region.
- Each region or grain consists of positive ions arranged in a regular pattern.
- The rows of atoms cause the electrons in the beam to be diffracted, just as a beam of light is diffracted when it passes through a slit.
- The electrons in the beam pass through the metal foil and are diffracted in certain directions only, as shown in the diagram above.
- They form a pattern of rings on a fluorescent screen at the end of the tube.
- Each ring is due to electrons diffracted by the same amount from grain of different orientations at the same angle to the incident beam.
- The beam of electrons is produced by attracting electrons form a heated filament wire to a positively charged metal plate, which has a small hole at the centre.
- Electrons that pass through the hole form the beam.
- The speed of the electrons can be increased by increasing the potential difference between the filament and the metal plate.
- Makes the diffraction rings smaller, and because of an increase in speed, the de Broglie wavelength gets smaller, so less diffraction occurs and the rings become smaller.
Electron microscope:
- Shorter wavelengths give smaller diffraction effects.
- Used in electron microscope.
- Diffraction effects blur detail on an image,
- If you want to resolve tiny detail in an image, a shorter wavelength is needed.
- Light blurs out detail more than “electron-waves” do.
- Therefore, the microscope can resolve finer detail than a light microscope.
- Therefore it can be used to look at tiny things like a strand of DNA.
